STAINLESS STEEL PRODUCTS
Utility Ferritics - Corrosion Resistance
Corrosion Resistance
From the report ‘Atmospheric Corrosion Testing in Southern Africa - Results of a Twenty Year Exposure Programme’ by BG Callaghan, Division of Materials Science and Technology, CSIR, the following graphs were constructed. The first graph shows the relative life of eight metals compared to mild steel in six different atmospheric environments. This can be summarised to give an average relative life of the different metals in atmospheric conditions and this is shown in the second graph
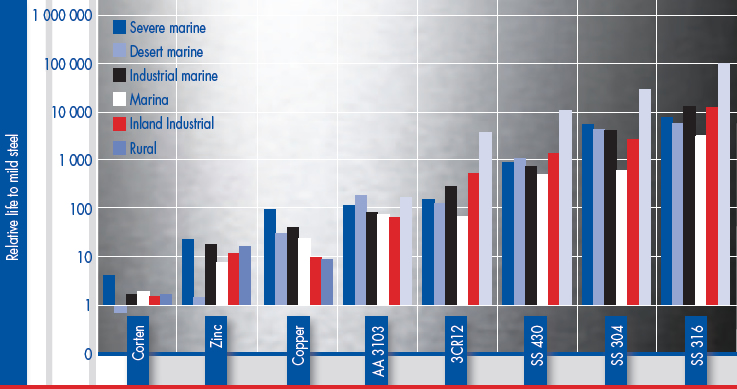
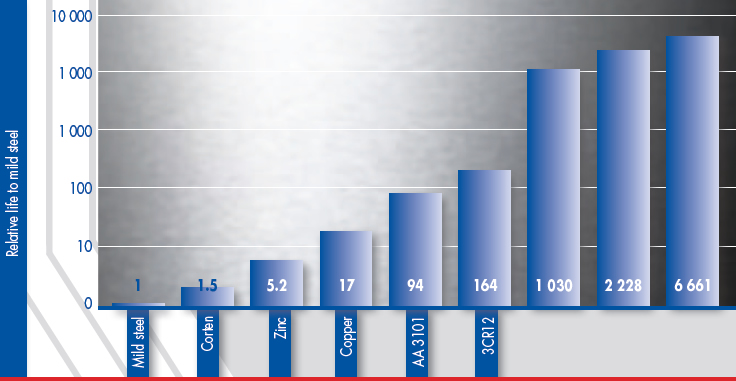
In appearance, all the metals showed discolouration at the more severe sites after 20 years. None of the metals were washed during the exposure programme and this clearly emphasises the importance of keeping stainless steel clean and that stainless steel is a LOW maintenance (not NO maintenance) option in atmospheric corrosion applications. 3CR12 showed some pitting, but the maximum pit depth after 10 years was 0.25mm.
The utility ferritics are significantly more corrosion resistant than mild or low alloy corrosion resistant steels. However, they have a lower corrosion resistance than the higher chromium standard ferritics. The utility ferritics should only be used in mildly corrosive conditions where aesthetics is not a prime requirement. A light surface patina or discolouration will form in most corrosive environments and this patina will, to some extent, retard further corrosion.
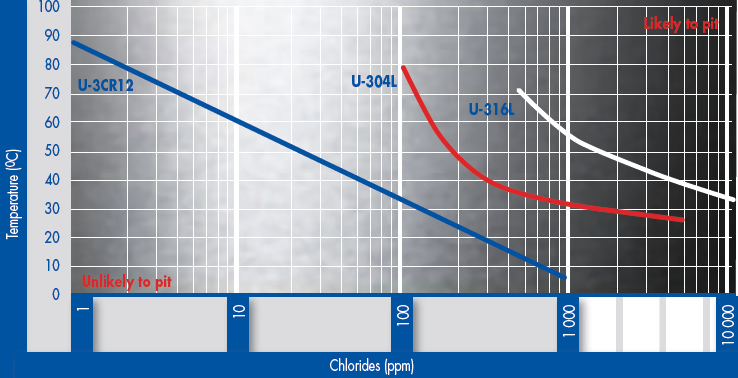
Pitting corrosion is possible in applications involving contact with chloride solutions, particularly in the presence of oxidising media. These conditions may be conducive to localised penetration of the passive surface film on the steel and a single deep pit may well be more damaging than a much greater number of relatively shallow pits. The diagram below shows the critical temperature for initiation of pitting (CPT) at different chloride contents (+350mV vs SCE).
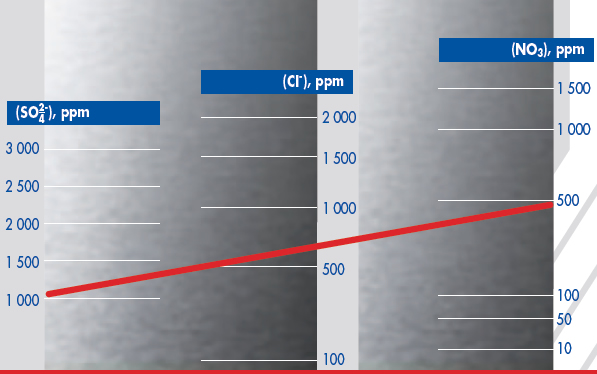
A model, shown in the second diagram, has been designed to predict the maximum concentration of chloride that can be permitted in water containing sulphate and nitrate ions before localised corrosion of 3CR12 takes place. A straight line, drawn between the concentrations of sulphate and nitrate, intersects the chloride axis at the maximum permissible chloride concentration for this water, at ambient temperature.
Applications | Chemical Compositions | Mechanical Properties | Physical Properties | Fabrication | Corrosion Resistance
